What Is Magnesium Iron Silicate Hydroxide? Things You Need To Know
Ever wondered what unseen forces and compounds shape the very ground beneath our feet? It's the intricate dance of minerals, and among them, magnesium iron silicate hydroxide plays a starring role, a crucial element in understanding our planet's composition and the processes that formed it.
Magnesium iron silicate hydroxide isn't a single mineral, but rather a chemical description that encompasses a family of minerals. Think of it as a category, like "fruit," under which many different varieties exist. These minerals, each with its unique crystal structure and properties, share the common thread of containing magnesium, iron, silicon, oxygen, and hydrogen (in the form of the hydroxide ion, OH-). This combination gives rise to a fascinating array of materials found in diverse geological settings.
| Aspect | Details |
|---|---|
| General Chemical Formula | Varies depending on the specific mineral, but generally involves (Mg,Fe)xSiyOz(OH)w |
| Common Minerals | Cummingtonite, Tremolite, Serpentine Group Minerals, Biotite, Phlogopite |
| Geological Occurrence | Metamorphic rocks, ultramafic rocks, altered igneous rocks |
| Uses | Industrial applications (brake linings, fireproof fabrics), pharmaceutical applications (dietary supplement, antacid), ornamental stone, soil amendment |
| Related Minerals | Olivine, Talc, Muscovite, Riebeckite, Magnesioriebeckite |
| Further Information | Mindat.org |
One prominent example is Cummingtonite. Chemically defined as (Mg, Fe 2+) 2 (Mg, Fe 2+) 5 Si 8 O 22 (OH) 2, this metamorphic amphibole is essentially a magnesium iron silicate hydroxide. It belongs to the amphibole group of minerals, characterized by their double-chain silicate structure. These minerals form when rocks undergo metamorphism, a process where heat and pressure alter the original mineral composition, leading to the creation of new minerals like cummingtonite.
- Brittany Ashton Holmes Now Life After Little Rascals Revealed
- Claudia Heffner Peltz Model Wife Philanthropist Full Story
Cummingtonite was first identified at Sterling Hill, New Jersey, though originally misidentified as Grunerite, according to Reilly's findings in 1983. It often appears alongside other metamorphic minerals and can sometimes be found in impressive crystal formations. Its appearance can range from fibrous to prismatic, and its color varies depending on the iron content. A sample of pyroxmangite might even show white pieces of cummingtonite intermingled within the structure, a testament to the complex interplay of mineral formation.
Another key group within the magnesium iron silicate hydroxide umbrella is the Serpentine group. These minerals, often described by the synonyms nagapashana and nagashma, are hydroxyl silicates. They are ubiquitous in geological systems. Serpentines are formed by the alteration of ultramafic rocks, particularly those rich in olivine and pyroxene. The alteration process, known as serpentinization, involves the addition of water to these minerals, transforming them into serpentine minerals.
The Serpentine group isn't a single mineral either, but a collection of minerals with similar structures and chemical compositions. Common serpentine minerals include antigorite, lizardite, and chrysotile. Chrysotile is particularly noteworthy because it is one of the main forms of asbestos. Serpentines are often found in regions with significant tectonic activity, where the Earth's crust is being deformed and altered. The term "serpentine" comes from the snake-like patterns often visible on the surface of these minerals.
- Adriana Limas Kids A Glimpse Into Her Family Life Updated
- Find Hindi Dubbed Movies Shows Online Your Guide
Olivine, though not strictly a magnesium iron silicate hydroxide because it lacks the hydroxide (OH-) group, is closely related and plays a crucial role in the formation of some of these minerals. Olivine, represented by the chemical formula (Mg,Fe)2SiO4, is a magnesium iron silicate. Its a nesosilicate or orthosilicate. As the primary component of the Earth's upper mantle, it's abundant in the Earth's subsurface. It weathers quickly when exposed to the surface. When olivine undergoes alteration, it can transform into serpentine minerals, illustrating the interconnectedness of mineral formation processes.
The chemical diversity within the magnesium iron silicate hydroxide family extends further. Biotite, with the formula K(Mg,Fe)3AlSi3O10(OH)2, is a potassium iron magnesium aluminum silicate hydroxide. Phlogopite, chemically kmg3alsi3o10(oh)2 (potassium magnesium aluminum silicate hydroxide), further displays this diversity. These are both micas and showcase how aluminum and potassium can also be incorporated into the structure of magnesium iron silicate hydroxide minerals. Biotite is typically brown to black, and phlogopite is pale yellow to golden brown.
Tremolite is another example a calcium magnesium iron silicate hydroxide. Its chemical formula reflects the presence of calcium alongside magnesium and iron. The amphibole minerals, as a broad group, follow the general formula XY2Z5(Si, Al, Ti)8O22(OH, F)2, where X, Y, and Z represent different cations (positively charged ions) that can occupy specific sites within the crystal structure. This formula allows for considerable variation in the chemical composition of amphibole minerals, resulting in a wide range of properties and occurrences.
Then there are the riebeckites. Riebeckite is the ferrous iron rich member, while magnesioriebeckite is the magnesium rich member. Magnesioriebeckites formula, Na 2 (Mg, Fe) 3 Fe 2 Si 8 O 22 (OH) 2 , is nearly identical to riebeckite. Thus, the properties of the two minerals are alike.
The silicates are complex compounds in which silicon and oxygen form an anion. This combines with cations such as aluminum and magnesium. Consider talc, a hydrated magnesium silicate, with the formula Mg 3 Si 4 O 10 (OH) 2. This highlights the importance of the hydroxide group in these minerals and its influence on their properties. Na 2 Ca(Mg, Fe) 5 Si 8 O 22 (OH) 2 is another, a sodium calcium magnesium iron silicate hydroxide.
Beyond their geological significance, magnesium iron silicates and magnesium iron silicate hydroxides possess a variety of industrial applications. Magnesium silicate, for example, is used to absorb moisture, prevent caking, and improve the feel of a product. It finds widespread use in various industries.
They are used in the pharmaceutical sector. They are used as a dietary supplement, as part of the formulation ingredients in drug production, and in antacid and antiulcer preparations. They are also a component of antiepileptic drugs, antifungal topical agents, and acne treatment. Serpentines, due to their fibrous nature, have been used in the past as asbestos, although this use is now heavily regulated due to health concerns. Many are used in industrial applications, including brake linings and fireproof fabrics. They are used as an ornamental stone.
Montmorillonite/smectite group, clays, and even some placed with the mica group are other examples of magnesium iron silicates or magnesium iron silicate hydroxides in action. They can be used as a toxic spill cleaner, a fire resistant material, soil additive, and other applications.
Its important to note the ongoing research into the precise properties and classifications of these minerals. For instance, in grunerite, the iron rich member, the ratio of mg\/(mg +fe) equals 0.00 to 0.29. But in magnesiocummingtonite, it is magnesium rich, the ratio equals 0.70 to 1.00. Cummingtonite by default has a ratio of 0.30 to 0.69. Therefore it is the most common member of the series.
The exploration of magnesium iron silicate hydroxide reveals a world of complexity and interconnectedness. These minerals, formed through geological processes spanning millennia, play a vital role in shaping our planet and influencing various aspects of our lives. Understanding their properties and occurrences is crucial for fields ranging from geology and materials science to environmental science and even medicine.
The name "Cummingtonite," some might argue, is rather peculiar. Its official name is magnesium iron silicate hydroxide, and it has the formula (mg,fe)7si8o22(oh)2. The name originates from Cummington, Massachusetts, USA, where it was first discovered.
Muscovite (hydrated aluminium potassium silicate[kal2(alsi3o10)(f, oh)2 ])and phlogopite (potassium magnesium aluminum silicate hydroxide) are the two major micas of commerce. Serpentines are the general designation of hydroxyl silicates and ubiquitous in many geological systems. Benchchem offers qualified products for magnesium iron silicate (cas no.2, magnesium iron silicate hydroxide.
(mg,fe)3si2o5(oh)4 is the magnesium iron silicate hydroxide class. For math, science, nutrition, and history, we compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. Additional recommended knowledge includes a correct test weight handling guide. Let us educate with a like, subscribe,and donation.
This page was last edited on 16 june 2021, at 15:41 (utc). Magnesium iron silicate hydroxide may refer to:
- Khabib Nurmagomedovs Wife All About Patimat 2024 Update
- Taurus Scorpio Friendship Compatibility Challenges More
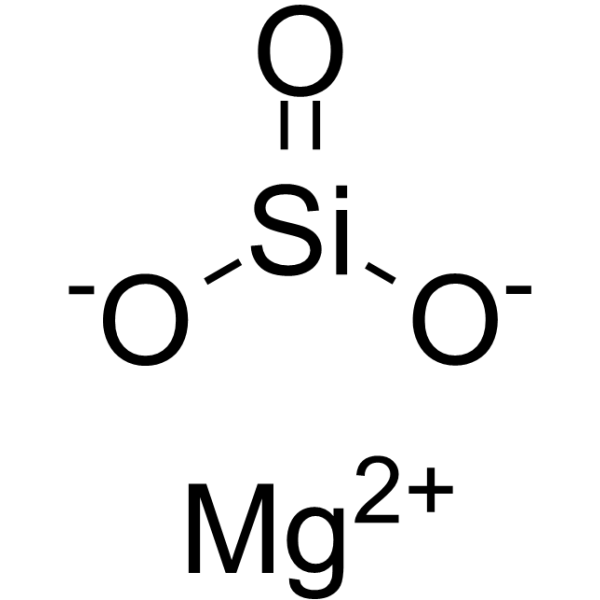
Magnesium silicate CAS 1343 88 0 Chemsrc

Magnesium Carbonate Hydroxide Formula at Gabriel Basser blog

Are you Magnesium Iron Silicate Hydroxide? Cuz you better be Fandom